An anchoring strategy leads to enhanced proton conductivity in a new metal–organic framework†
Abstract
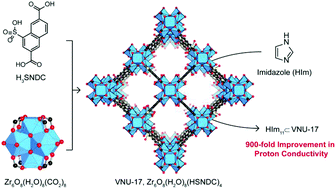
A new Zr(IV)-based metal–organic framework, termed VNU-17 [Zr6O8(H2O)8(HSNDC)4, where HSNDC2− = 4-sulfonaphthalene-2,6-dicarboxylate], was designed to enhance proton conductivity through the incorporation of Brønsted acid functionalities. The resulting VNU-17 framework adopted the bcu topology and the structure was highlighted by densely packed sulfonic acid groups lining 6 Å channels. Following this, the internal void space of VNU-17 was engineered to further enhance proton conductivity, in which the proton transfer agent, imidazole (HIm), was anchored within the structure in two different loadings. Remarkably, the material with the highest HIm load, HIm11⊂VNU-17, exhibited a 900-fold enhancement in proton conductivity (5.9 × 10−3 S cm−1 at 85% RH and 70 °C) in comparison with the parent VNU-17 structure and was shown to maintain its performance over at least 40 h. These findings demonstrate the potential for systematically fine-tuning such materials for use as electrolyte materials in proton exchange membrane fuel cells.


 Please wait while we load your content...
Please wait while we load your content...