Metal–organic gels of silver salts with an α,β-unsaturated ketone: the influence of anions and solvents on gelation†
Abstract
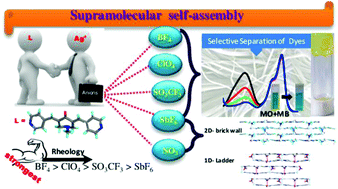
A bis-pyridyl substituted α,β-unsaturated ketone, namely 1-methyl-3,5-bis-pyridin-4-ylmethylene-piperidin-4-one, L, was shown to form metal organic gels (MOGs) with silver salts. The presence of anions such as tetrafluoroborate, perchlorate, triflate and hexafluoroantimonate resulted in the formation of MOGs in various solvents. The MOGs exhibit well-developed nanofibrillar networks composed of intertwined fibers and are characterized by field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), Fourier transform infrared (FT-IR) spectroscopy, rheology and powder X-ray diffraction (PXRD) techniques. The gelation was studied in as many as twenty organic solvents. Among all the solvents used, benzonitrile was found to promote the gelation of L with as many as four silver salts, namely BF4 (MOG-1), ClO4 (MOG-2), CF3SO3 (MOG-3) and SbF6 (MOG-4). All these xerogels were found to be polycrystalline in nature as confirmed by PXRD. FESEM images of the xerogel of MOG-2 revealed that it exhibits spherical morphology consisting of fibrils. Rheological studies indicate that MOG-1 is the strongest gel among all four and the rigidity order follows BF4 > ClO4 > CF3SO3 > SbF6. Furthermore, the xerogels were found to exhibit selective dye adsorption and separation. The information on interactions and structures involved in the gelation of solvents was obtained by the single crystal structure determination of Ag(I) complexes of L with NO3 and SbF6 anions.

 Please wait while we load your content...
Please wait while we load your content...