MOF-templated nitrogen-doped porous carbon materials as efficient electrocatalysts for oxygen reduction reactions†
Abstract
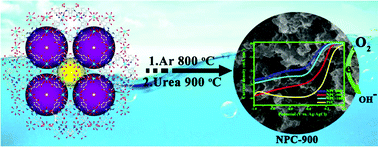
MOF-templated nitrogen-doped porous carbon materials (NPCs) have been prepared by employing the previously reported MOF, [(CH3)2NH2]6[Ni(H2O)6]3{Ni6(η6-TATAT)4(H2O)12}, as a precursor, which can act as efficient electrocatalysts for oxygen reduction reactions (ORRs). The carbonized MOF acts as the carbon source, and urea is employed as an additional nitrogen source. The urea modified carbon materials, carbonized at 800 °C, 900 °C and 1000 °C under Ar atmosphere, all show electrocatalytic activity for ORRs; the material carbonized at 900 °C exhibits a more appropriate nitrogen content, robust pore structure and an excellent degree of graphitization. In alkaline media, NPC-900 exhibits superior catalytic activity (the onset and half-wave potentials are −0.06 V and −0.23 V vs. Ag/AgCl, respectively), excellent long-term durability and outstanding methanol tolerance compared with the commercial Pt/C catalyst.


 Please wait while we load your content...
Please wait while we load your content...