Selective ethylene oligomerisation using supported tungsten mono-imido catalysts†
Abstract
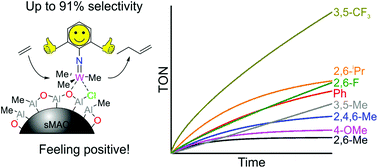
A series of substituted phenyl mono-imido complexes of the type W(NR)Cl4(THF) (R = C6H5, 2,6-Me-C6H3, 3,5-Me-C6H3, 2,4,6-Me-C6H2, 4-OMe-C6H4, 2,6-F-C6H3 and 3,5-CF3-C6H3) have been synthesised and characterised. Reaction of these complexes with solid polymethylaluminoxane (sMAO) leads to immobilisation and in situ methylation of the chloride positions on the surface of the support. Reaction of W(NR)Cl4(THF) with trimethylaluminium (TMA) yields the trimethyl complexes W(NR)Me3Cl. Immobilisation of the isotopically labelled W{N(2,6-F-C6H3)}(13CH3)3Cl on sMAO furnished the supported complex with two identifiable methyl resonances in the 13C–{1H} solid state CPMAS spectrum (45 and 56 ppm), with the latter matching the unsupported complex, confirming retention of the structure on the surface. The sMAO-supported complexes (W : Al = 1 : 150) were tested for their propensity to dimerise ethylene (1 bar) in d6-benzene at 100 °C and compared with the previously reported sMAO-W{N(2,6-iPr-C6H3)}Cl4(THF) (sMAO-1.a). Complexes with electron deficient imido groups were shown to be the most active, and increased steric bulk in the ortho positions is also an important factor, with sMAO acting as a support, scavenger and activator. sMAO-W{N(3,5-CF3-C6H3)}Cl4(THF) was the most active, demonstrating a turnover frequency of 5.65 molC2H4 mol−1W h−1 and a selectivity towards 1-butene of 91% after 8 h.


 Please wait while we load your content...
Please wait while we load your content...