A nickel–borate–phosphate nanoarray for efficient and durable water oxidation under benign conditions†
Abstract
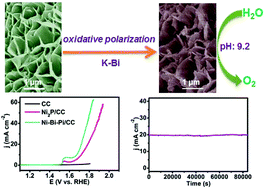
The development of earth-abundant electrocatalysts for efficient water oxidation under moderate conditions is highly desired but still a big challenge. In this communication, we demonstrate the topotactic conversion of a nickel phosphide nanoarray on carbon cloth into a nickel–borate–phosphate nanoarray (Ni–Bi–Pi/CC) by oxidative polarization in potassium borate water. When used as a 3D water oxidation catalyst, such Ni–Bi–Pi/CC shows high activity with a geometrical catalytic current density of 10 mA cm−2 at an overpotential of only 440 mV in 0.1 M K–Bi, rivaling the performances of the reported Ni-containing catalysts operated under benign conditions. Notably, this electrode also demonstrates strong long-term electrochemical durability with 100% Faradaic efficiency for oxygen evolution. All these features promise its use as an attractive low-cost catalyst electrode in water-splitting devices for mass production of hydrogen fuels under environmentally friendly conditions.


 Please wait while we load your content...
Please wait while we load your content...