A microporous Mg2+ MOF with cation exchange properties in a single-crystal-to-single-crystal fashion†
Abstract
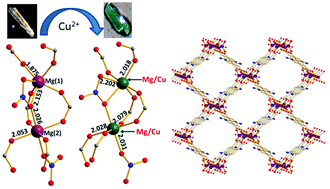
We report herein a new alkaline earth metal ion organic framework [Mg2(NH2BDC)2(HNO3)]·9H2O (AEMOF-7), which shows a 3-D microporous structure with several unusual features, such as the rare trigonal prismatic coordination geometry of one of the crystallographically unique Mg2+ centers and the existence of a bridging HNO3 ligand. The H+ ions of the HNO3 ligand are dissociable as demonstrated via proton conductivity measurements. AEMOF-7 displays relatively high selectivity for CO2vs. CH4 and negligible N2 uptake. Interestingly, this compound was found to be capable of single-crystal-to-single-crystal (SCSC) exchange of Mg2+ by Cu2+ ions, which was observed for the first time in a MOF material. AEMOF-7 is also luminescent and its photophysical properties were investigated via solid state UV-Vis, steady-state and time-resolved luminescence studies.
- This article is part of the themed collection: In honour of Mercouri G. Kanatzidis for his contributions to Inorganic Chemistry for over 30 years


 Please wait while we load your content...
Please wait while we load your content...