Phase and morphology evolution during the solvothermal synthesis of VO2 polymorphs†
Abstract
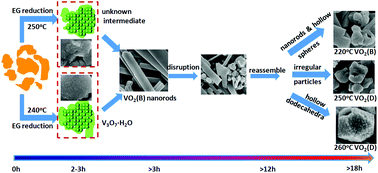
The phase and morphological features of materials are often tunable by adjusting the reaction parameters of solvothermal synthesis but this versatility also poses a challenge for preparing materials with a desired phase and morphology if the behaviors of phase and morphological evolution during the solvothermal synthesis are not known. In this work, the formation and growth of VO2 nanomaterials in the solvothermal systems via the reduction of V2O5 by ethylene glycol (EG) were investigated by in situ powder X-ray diffraction (PXRD). The results show that both fast and slow heating produce the same VO2(B) final product but the phase evolution during the synthesis is very sensitive to the heating rate. Fast heating (10 °C min−1) involves an unknown intermediate while V3O7·H2O is the intermediate phase at slow heating (2 °C min−1). The formation mechanism was employed to design the synthesis of VO2(B) nanorods and the phase transformation paths were verified by large-scale batch synthesis. Furthermore, ex situ PXRD and SEM were employed to follow the structure and morphology evolution during growth. This research indicates that in situ PXRD, as a powerful tool to monitor the whole reaction process and to collect information such as phase evolution and the fate of the transient intermediates, can be used to direct the controlled synthesis of materials.

 Please wait while we load your content...
Please wait while we load your content...