Four-connected metal–organic frameworks constructed by tetracarboxylate acid-based ligands†
Abstract
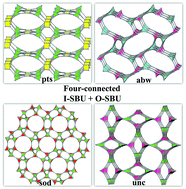
Three tetracarboxylate acid ligands, 5,5′-(1H-1,2,3-triazole-1,4-diyl)diisophthalic acid (H4TADIP), 5-(bis(4-carboxybenzyl)amino)isophthalic acid (H4BCBAIP), and 5-(3,5-dicarboxybenzyloxy)isophthalic acid (H4DBIP) have been reacted with Mn(II), Co(II) and In(III) salts to construct four new metal–organic frameworks, [Mn2(TADIP)(DMF)3]·2DMF·3H2O (1), [Co2(TADIP)(DEF)3]·0.5DEF (2), [Mn2(BCBAIP)(DMF)3]·7.5DMF (3), and (Me2NH2)[In(DBIP)]·3DMF·7H2O (4) under solvothermal conditions. Due to the rotation ability of these ligands that exhibit a quadrangle or tetrahedral geometry, these compounds show diverse three-dimensional four-connected topologies. Compound 1 is composed of binuclear Mn(II) units and quadrangle type ligand H4TADIP with pts topology, while compound 2 possesses a zeolitic abw topology constructed from the 4-connected tetrahedral type ligand H4TADIP and binuclear Co(II) units. Compound 3 is a 3D structure possessing a zeolitic sod topology from the coordination of binuclear Mn(II) units with the tetrahedral type ligand H4BCBAIP. Compound 4 is an anionic framework constructed from tetrahedral type ligand H4DBIP and In(III) ions that has a topology of unc. Variable temperature magnetic susceptibility measurements show that compound 1 exhibits antiferromagnetic behavior. The selective dye-adsorption and luminescent properties of compound 4 have been studied in detail.

 Please wait while we load your content...
Please wait while we load your content...