Metal-ion controlled solid-state reactivity and photoluminescence in two isomorphous coordination polymers†
Abstract
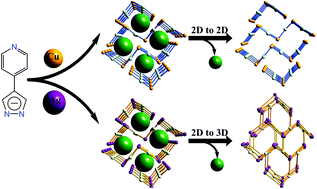
Reactions of [M(NH3)2]OH (M = Ag and Cu) and 4-(1H-pyrazol-4-yl)pyridine (Hpypz), using p-xylene (C8H10) as the template, gave isomorphous two-dimensional porous metal azolate frameworks [Ag2(pypz)2]·0.5C8H10 (MAF-36(Ag), 1·g) and [Cu2(pypz)2]·0.5C8H10 (MAF-36(Cu), 2·g). Powder and single-crystal X-ray diffraction studies showed that, upon guest removal, 1·g transformed into a three-dimensional nonporous framework [Ag6(pypz)6] (1′), while 2·g can retain its porous two-dimensional structure. Depending on the metal ion, framework flexibility/rigidity, and/or porosity, 1·g and 2·g, as well as their guest-free derivatives, showed distinctly different photoluminescence and guest-responsive behaviors.

 Please wait while we load your content...
Please wait while we load your content...