Synthesis of aromatic-doped polycaprolactone with tunable degradation behavior†
Abstract
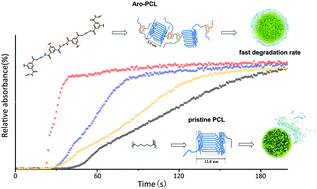
A novel aromatic-doped polycaprolactone (Aro-PCL) material was synthesized through a facile PCL aminolysis-condensation polymerization incorporating the aromatic moiety to PCL chain and assessed by focusing on the dynamic aggregation and crystalline microdomains associated with the in vitro degradation properties, mechanical performance and biocompatibility. Characterizations by FTIR, NMR, UV-vis, DSC, WAXD, SAXS, POM, etc. illustrated that the incorporated aromatic and amine segments facilitated the formation of many low-oriented crystalline domains along the aliphatic chains. The results indicated that the evolution of mechanical performance is significantly associated with the chemical structure and the crystallization behavior of polymers. The Aro-PCL films possessed fast degradation rate, excellent biocompatibility and improved mechanical performance including augmented elongation at break, elastic modulus and radial strength. In particular, the Aro-PCL polymer films showed decrease in molecular weight at the beginning of hydrolytic degradation while maintaining mechanical support and stable mass. The Aro-PCL polymer has promising applications as a degradable biomaterial.


 Please wait while we load your content...
Please wait while we load your content...