Opposite-charge repulsive cation and anion pair cooperative organocatalysis in ring-opening polymerization†
Abstract
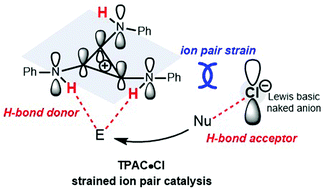
Cations and anions attract each other by electrostatic force to form an ion pair. However, repulsion between cations and anions does exist. There are few examples of repulsive “ion pair strain” where catalysis by a strained ion pair is absent. Here, we describe substituted cyclopropenium, the minimal Hückel aromatic ring, which when mounted with PhNH groups on the positive cyclopropenium core C3, behaves as an electron-rich cation to repel its counter negative anion. The formal positive charge on C3 turns the NH moiety into a strong H-bond donor (HBD) and the weakly coordinating chloride exhibits strong H-bond acceptor (HBA) character. The strained ion pair composed of a HBD and a HBA displays cooperative organocatalysis in ring-opening polymerizations of δ-valerolactone initiated with benzyl alcohol. A cooperative catalysis mechanism of tris(phenylamino)cyclopropenium as a HBD and chloride as a HBA was elucidated.


 Please wait while we load your content...
Please wait while we load your content...