A switch from anionic to bifunctional H-bonding catalyzed ring-opening polymerizations towards polyether–polyester diblock copolymers†
Abstract
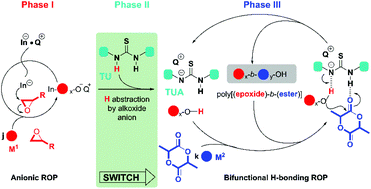
The copolymerization of structurally distinct epoxides and cyclic esters offers polyether-block-polyester copolymers which are valuable synthetic materials. However, the anionic ring-opening polymerization (ROP) of glycidyl ethers is not usually compatible with the subsequent ROP of lactide (LA). We designed a switchable approach composed of the tetrabutylammonium fluoride initiated ROP of glycidyl phenyl ether (GPE), followed by the addition of thiourea (TU) to initiate the ROP of LA. The active alkoxide chain end of the first ROP was turned into an alcohol, and the TU was turned into a thioureate anion (TUA). The TUA promoted the ROP of LA from the alcohol via bifunctional H-bonding catalysis to full monomer conversion in a controlled/living manner. Poly(GPE-b-LA) with precise molecular weights and narrow dispersities (Đ, 1.13 to 1.19) were prepared at room temperature in solution. 1H NMR titrations suggested the formation of the TUA and its coordination with chain end hydroxyl; this played key roles in the switch of ROPs and H-bonding catalyzed ROP of LA. The anionic to H-bonding switchable catalysis showed a general and simple protocol to copolymerize epoxides and cyclic esters into diblock copolymers.


 Please wait while we load your content...
Please wait while we load your content...