Polymers with acyl-protected perthiol chain termini as convenient building blocks for doubly responsive H2S-donating nanoparticles†
Abstract
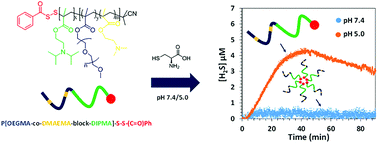
H2S-releasing polymers with an acyl-protected perthiol chain terminus were prepared using a simple, high yielding end-group modification process. Specifically, benzodithioate-terminated poly(oligoethylene glycol methyl ether) methacrylate (POEGMA) was first converted to pyridyl-2-disulfide-terminated polymer, after which a thiol–disulfide exchange reaction with thiobenzoic acid yielded an acyl protected perthiol at the chain terminus. The same approach was successfully applied to a hydrophilic–hydrophobic block polymer, P[OEGMA-block-n-butyl methacrylate], and a pH-responsive block copolymer, P[OEGMA-co-N,N-(dimethylamino) ethyl methacrylate-block-N,N-(diisopropylamino)ethyl methacrylate]. All polymers were shown to release H2S when exposed to thiol (L-cysteine), with release rate dependent on polymer structure. In the case of the pH-responsive block copolymer there was minimal release of H2S under conditions where the polymers were micellised, whereas there was rapid, sustained release when the block copolymers were in unimeric form. These materials were shown to increase the intracellular concentration of H2S when applied to HEK cells, and may be useful for interrogating localized delivery of H2S.


 Please wait while we load your content...
Please wait while we load your content...