Analysis of the reaction mechanism of the thiol–epoxy addition initiated by nucleophilic tertiary amines
Abstract
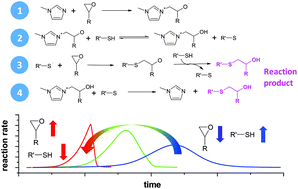
A kinetic model for thiol–epoxy crosslinking initiated by tertiary amines has been proposed. The kinetic model is based on mechanistic considerations and it features the effect of the initiator, hydroxyl content, and thiol–epoxy ratios. The results of the kinetic model have been compared with data from the curing of off-stoichiometric formulations of diglycidyl ether of bisphenol A (DGEBA) crosslinked with trimethylolpropane tris(3-mercaptopropionate) (S3) using 1-methylimidazole (1MI) as the initiator. The model has been validated by fitting the kinetic parameters to the experimental data under a variety of reaction conditions. In spite of the experimental uncertainty and model assumptions, the main features of the curing kinetics are correctly described and the reaction rates are quantitatively reproduced.


 Please wait while we load your content...
Please wait while we load your content...