Four-fold increase in epoxide polymerization rate with change of alkyl-substitution on mono-μ-oxo-dialuminum initiators†
Abstract
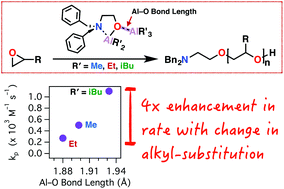
We present an improvement in the rate, utility, and mechanistic understanding of mono-μ-oxo-dialuminum initiators for epoxide ring-opening polymerization based on investigation of a homologous series of trialkylaluminum adducts of (2-dibenzylamino)ethoxy-dialkylaluminum (TAxEDA) [(AlR3)·(O(AlR2)CH2CH2N(Bn)2), with R = Me, Et, iBu]. Using in situ FTIR spectroscopy of neat AGE polymerizations, we determined that the isobutyl-substituted TAxEDA (iBu-TAxEDA) exhibited a propagation rate constant (kp) of 1.100 ± 0.022 × 10−3 M−1 s−1, which was twice that of the methyl-functional TAxEDA (Me-TAxEDA) (kp = 0.500 ± 0.011 × 10−3 M−1 s−1) and four times that of ethyl-functional TAxEDA (Et-TAxEDA) (kp = 0.270 ± 0.003 × 10−3 M−1 s−1). The dative R3Al–O bond length in the mono-μ-oxo-dialuminum was longest for the iBu-TAxEDA (1.93 Å) and shortest for the Et-TAxEDA (1.88 Å). Consistent with a previously proposed mechanism for TAxEDA-initiated polymerization, the increased Al–O bond length may accommodate more-facile coordination and enchainment of the monomer by separation of the Al–O interaction leading to an increased polymerization rate. The generality of the improved iBu-TAxEDA was supported by polymerization of a range of monomer substrates such as propylene oxide (PO), butylene oxide (BO), epichlorohydrin (ECH), allyl glycidyl ether (AGE), propargyl glycidyl ether (PGE), and adamantylmethyl glycidyl ether (AMGE). In all cases investigated, the triisobutyl-functional TAxEDA (iBu-TAxEDA) represented an improved initiator for epoxide polymerization.


 Please wait while we load your content...
Please wait while we load your content...