ADMET polymerization of α,ω-unsaturated glycolipids: synthesis and physico-chemical properties of the resulting polymers†
Abstract
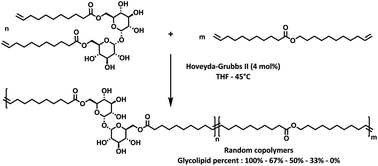
Trehalose diesters exhibiting α,ω-unsaturation are glycolipids which can be easily polymerized by ADMET (acyclic diene metathesis) polymerization. In this paper, enzymatic esterification was performed to selectively esterify primary hydroxyl groups of trehalose (6 and 6′-positions) with vinyl undecenoate. The vinyl ester was beforehand obtained by palladium-catalyzed transesterification of undecenoic acid with vinyl acetate. The resulting trehalose diundecenoate was homopolymerized and copolymerized with undecenyl undecenoate in order to obtain random copolymers with different compositions. The synthesis of such copolymers was confirmed by 1H NMR spectroscopy and size exclusion chromatography (SEC). Their solid-state phase separation was investigated by DSC and X-ray scattering as a function of temperature and their solution self-assembly was investigated by dynamic light scattering (DLS) in water.


 Please wait while we load your content...
Please wait while we load your content...