The stirring rate provides a dramatic acceleration of the ultrafast interfacial SET-LRP in biphasic acetonitrile–water mixtures
Abstract
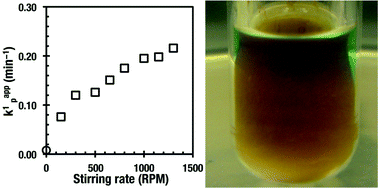
The influence of the stirring rate on the SET-LRP of methyl acrylate and n-butyl acrylate catalyzed with a Cu(0) wire in methanol and with Cu(0) generated by the reduction of Cu(II)Br2 with NaBH4 by two different methodologies in biphasic acetonitrile–water mixtures was investigated. In all cases tris(2-dimethylaminoethyl)amine (Me6-TREN) was used as a ligand and the polymerizations were carried out at 25 °C. No effect of the stirring rate on the rate of SET-LRP was observed in methanol when the Cu(0) wire was used as a catalyst and the reaction mixture exhibited a single homogeneous phase. However, a dramatic acceleration of the already ultrafast interfacial SET-LRP was observed in biphasic acetonitrile–water mixtures for both methodologies employed for the generation of Cu(0) directly in the reaction mixture. No increase in the dispersity of the resulting polymers was observed when the rate of the reaction was increased by stirring. This is contrary to the rate of polymerization increased by catalyst concentration. Mechanistic hypothesis for this increase in the rate of SET-LRP in biphasic acetonitrile–water systems was advanced. This catalytic-like effect of the stirring rate is expected to be of interest for both laboratory and technological applications.


 Please wait while we load your content...
Please wait while we load your content...