Facile synthesis of oligo(3-hexylthiophene)s conductive wires with charge-transfer functions†
Abstract
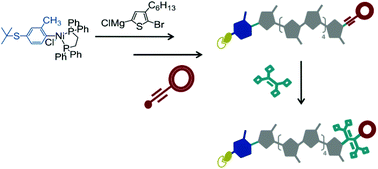
A series of fully conjugated oligo(3-hexylthiophene)s bearing different starting- and end-groups have been synthesized by means of externally initiated Kumada catalyst-transfer polymerization (KCTP) and Grignard Metathesis Polymerization (GRIM). These kinds of oligomers’ starting- and end-groups include tert-butyl protected thiols to be used for binding of oligomers to gold electrodes and tetracyanobutadiene-based donor–acceptor (DA) end-groups, such as dimethylaniline-tetracyanobutadiene (DMA-TCBD) and ferrocene-tetracyanobutadiene (Fc-TCBD), introduced to control the charge transport through the oligomers. The DMA-TCBD and Fc-TCBD end groups were incorporated by means of a Diederich-type click transformation of appropriately end-terminated oligo(3-hexylthiophene)s. The efficiency of the end-group functionalization was comprehensively assessed by NMR spectroscopy and MALDI-TOF spectrometry whereas the redox activities of the DA end-groups were examined by cyclic voltammetry. KCTP showed a much superior performance compared to GRIM in the introduction of a desirable end-group functionality. The thus-prepared conjugated oligomers are attractive materials for application in molecular electronics which will be explored in future studies.

 Please wait while we load your content...
Please wait while we load your content...