Superbase catalyzed regio-selective polyhydroalkoxylation of alkynes: a facile route towards functional poly(vinyl ether)s†
Abstract
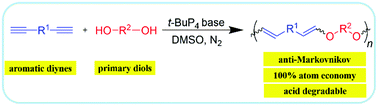
The polyhydroXations, such as polyhydrothiolation and polyhydroamination, of alkynes have been well-established. However, the polyhydroalkoxylation is rarely reported. In this paper, the polyhydroalkoxylation of aromatic diynes was successfully developed in the presence of a superbase, phosphazene base (t-BuP4). A series of soluble and regio-regular anti-Markovnikov additive poly(vinyl ether)s with high molecular weights (Mw up to 40 600) were obtained in high yields (up to 99%). All the polymers are thermally and morphologically stable. The tetraphenylethene (TPE)-containing P1a2a, P1a2b and P1a2c showed unique aggregation-enhanced emission (AEE) characteristics, and their aggregates could be used for explosive detection with a superamplification quenching effect. The poly(vinyl ether)s are degradable under acidic conditions. Thus, this work not only established a new polymerization but also generated a series of functional polymeric materials that are potentially applicable in optoelectronic and biomedical fields.


 Please wait while we load your content...
Please wait while we load your content...