A cellulose-based chiral fluorescent sensor for aromatic nitro compounds with central, axial and planar chirality†
Abstract
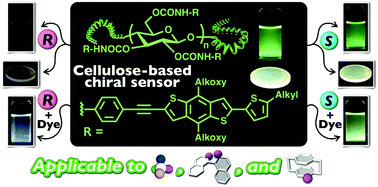
Chiral sensing using fluorescent responses as output signals is an attractive technique for enantiodifferentiation in terms of its rapidity, high sensitivity, simplicity and high-throughput ability. However, because the reported sensors can only be applied to a limited type of chiral molecule (mainly compounds with a chiral center), it is still a great challenge to develop a powerful fluorescent sensor applicable to various types of chirality. Herein, we synthesized a novel chiral fluorescent sensor (Ce-3) containing a benzo[1,2-b:4,5-b′]dithiophene-based π-conjugated group as a fluorescent signaling unit through a two-step polymer reaction, including carbamoylation and cross-coupling reactions, using microcrystalline cellulose as a starting material. The enantioselective fluorescence response of this modified cellulose to aromatic nitro compounds was investigated in solution and in the solid state. Ce-3 exhibited enantioselective fluorescence quenching for a wide range of aromatic nitro compounds with central, axial and planar chirality. Visual chiral detection based on a change of the visible emission color was also achieved with Ce-3 in conjunction with anthracene as an achiral fluorescent dye. A corresponding model molecule did not show any marked sensing ability, suggesting that the regular higher-order structure of Ce-3 plays a key role in this efficient chiral sensing.


 Please wait while we load your content...
Please wait while we load your content...