CO2-Driven stereochemical inversion of sugars to create thymidine-based polycarbonates by ring-opening polymerisation†
Abstract
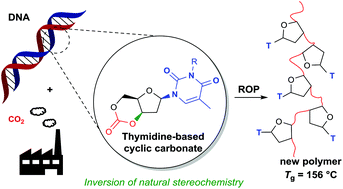
The development of biodegradable polymers from renewable resources is vital in addressing the dependence of plastics on petroleum-based feedstocks and growing ocean and landfill waste. Herein, both CO2 and natural sugar diols are utilised as abundant, safe and renewable building blocks for the synthesis of degradable and biocompatible aliphatic polycarbonates. Despite a strong potential for advanced polymer properties, inspired by Nature's supramolecular base-pairing, polycarbonates from the sugar components of DNA, 2′-deoxyribonucleosides have been limited by the inability of phosgene derivatives to form the cyclic carbonate monomers that would allow for controlled ring-opening polymerisation. CO2 insertion at 1 atm pressure into methylated thymidine 2′-deoxyribonucleoside, facilitated by organic base 1,8-diazabicyclo-[5.4.0]-undec-7-ene, affected an intramolecular SN2-like displacement of a tosyl leaving group to yield the cyclic carbonate by stereochemical inversion. Organocatalytic ring-opening polymerisation proceeded rapidly in solution resulting in high monomer conversions of 93% and number-average molecular weights, substantially greater and more controlled than via polycondensation routes. The thermodynamic parameters of the polymerisation (ΔHp = −12.3 ± 0.4 kJ mol−1 and ΔSp = −29 ± 1.1 J mol−1 K−1) were determined from the equilibrium monomer conversions over a temperature range of 0 to 80 °C and pseudo-first order kinetics demonstrated. The amorphous thymidine-based polycarbonates exhibited high glass transition temperatures of 156 °C and were found to be highly degradable to the constituent diol under basic aqueous conditions. Static water contact angle measurements and cell studies with MG-63 cell line indicated slightly hydrophilic and biocompatible materials, promising for tissue-engineering applications. The novel, CO2-driven approach to cyclic carbonate synthesis represents a means of expanding the scope of sugar-based monomers for tailored material properties derived from natural products.


 Please wait while we load your content...
Please wait while we load your content...