Synergetic effect of the epoxide functional groups in the photocatalyzed atom transfer radical copolymerization of glycidyl methacrylate
Abstract
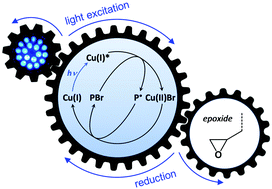
Methyl methacrylate (MMA) and glycidyl methacrylate (GMA) were copolymerized by photocatalyzed atom transfer radical polymerization under the visible light irradiation of a compact blue LED lamp, using bis(1,10-phenanthroline)copper(I) as the photocatalyst. The polymerization was found to be all the more fast that the molar fraction of GMA was high. The same effect was observed when GMA was replaced by cyclohexene oxide (CHO) as a non-copolymerizable epoxide-containing compound. These results suggested that the epoxide functional group acted as a reducing agent in both cases, contributing to a faster regeneration of the activator form of the catalyst. This assumption was confirmed by UV-visible spectroscopy measurements, which evidenced a faster conversion of Cu(II) to Cu(I) in the presence of epoxide functional groups. Due to their large excess compared to the catalyst, no degradation of the epoxides was detected during the copolymerization of MMA and GMA, which thus provided well-defined poly(MMA-stat-GMA) copolymers as precursors for further functionalization. The statistical distribution of the epoxide side groups could also be controlled through sequenced additions of the comonomers, in a direct “one-pot” approach.

 Please wait while we load your content...
Please wait while we load your content...