Biodegradable hyperbranched polyether-lipids with in-chain pH-sensitive linkages
Abstract
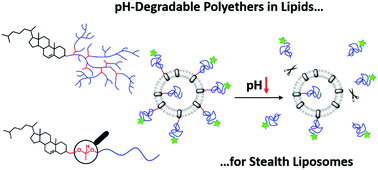
Hyperbranched polyether-based lipids with cleavable acetal units were obtained via copolymerization of the epoxide inimer 1-(glycidyloxy)ethyl ethylene glycol ether (GEGE) and glycidol, using anionic ring-opening polymerization. Cholesterol-linear polyglycerol (Ch-linPG) was used as a macroinitiator, resulting in branched polyethers with an adjustable amount of acid-cleavable units. Random copolymerization led to Ch-P(GEGEx-co-Gy) copolymers, whereas sequential copolymerization provided access to Ch-P(GEGEx-b-Gy) amphiphiles. The amount of GEGE was varied between 8–49 mol% of the total amount of monomer units. In addition, hyperbranched polyethers with a single acetal unit were prepared using glycol-1-(cholesteryloxy)ethylether as an initiator for the polymerization of allyl glycidyl ether (AGE) in bulk. Subsequent thiol–ene coupling of mercaptoethanol resulted in the hydroxyl functional macroinitiator used for the polymerization of glycidol. The novel polyether-based lipids were characterized in detail by 1H NMR spectroscopy and size exclusion chromatography, revealing narrow to moderate molecular weight distributions. Degradation was achieved at pH 2 in a proof-of-principle experiment. Acid-triggered shedding of liposomes was proven using the linear analogue α-(1-(cholesteryloxy)ethoxy)-ω-hydro-PEG-CH2-C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH with one cleavable group and a fluorescence label, Atto 488 azide. Investigation of the acetal-cleavage under neutral and acidic pH (7.4–2.0) via fluorescence spectroscopy was carried out.
CH with one cleavable group and a fluorescence label, Atto 488 azide. Investigation of the acetal-cleavage under neutral and acidic pH (7.4–2.0) via fluorescence spectroscopy was carried out.

 Please wait while we load your content...
Please wait while we load your content...