100% hyperbranched polymers via the acid-catalyzed Friedel–Crafts aromatic substitution reaction†
Abstract
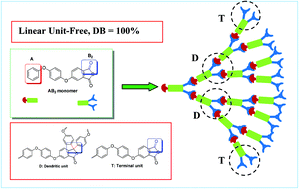
A highly efficient and benign strategy to synthesize hyperbranched polymers with a degree of branching of 100% was presented via a Friedel–Crafts aromatic substitution reaction of an AB2 monomer, 5-(4-phenoxyphenoxy)isobenzofuran-1,3-dione, in the presence of trifluoromethanesulfonic acid. The molecular weight (Mw: 66.1–294.8 kDa) and the polydispersity (close to 2.0) can be tuned by controlling polymerization conditions. It makes it convenient to tailor hyperbranched polymers for potential application in functional materials.

 Please wait while we load your content...
Please wait while we load your content...