A star-shaped amphiphilic block copolymer with dual responses: synthesis, crystallization, self-assembly, redox and LCST–UCST thermoresponsive transition†
Abstract
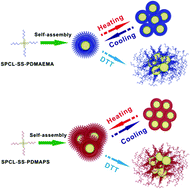
Star-shaped amphiphilic poly(ε-caprolactone)-SS-poly(N,N-dimethylaminoethyl methacrylate) (SPCL-SS-PDMAEMA) was synthesized by a combination of ring-opening polymerization (ROP), dicyclohexylcarbodiimide (DCC) reaction and atom transfer radical polymerization (ATRP). After the quaternization reaction of the PDMAEMA segment with excess 1,3-propane sultone, poly(ε-caprolactone)-SS-poly(3-dimethyl(methacryloyloxyethyl) ammonium propane sulfonate) (SPCL-SS-PDMAPS) was obtained. The star-shaped structure of the copolymers and the presence of the amorphous PDMAEMA and PDMAPS in copolymers decreased or even completely suppressed the crystallizability of PCL in copolymers. These copolymers could self-assemble into spherical micelles in water. The thermoresponsive micelle solutions showed transition from a lower critical solution temperature (LCST) of SPCL-SS-PDMAEMA to an upper critical solution temperature (UCST) of SPCL-SS-PDMAPS, indicating that the LCST–UCST transition of the micellar solutions can be accomplished by the transition of PDMAEMA to PDMAPS. Moreover, a redox-responsive investigation showed that the distributions of hydrodynamic radius (Rh) broadened with the emergence of aggregates when DL-dithiothreitol (DTT) was added into the micellar systems, since DTT would cause the breakage of disulfide bonds linking PCL and the PDMAEMA/PDMAPS blocks in copolymers.

 Please wait while we load your content...
Please wait while we load your content...