Blood compatibility of heparin-inspired, lactose containing, polyureas depends on the chemistry of the polymer backbone†
Abstract
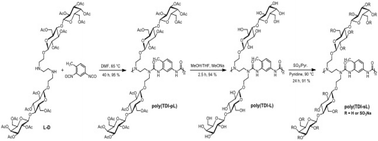
The influence of polymer chemical structure and degree of sulfation of polyurea glycopolymers on blood compatibility is reported. Polymers were prepared using lactose-containing diamines with either toluene 2,4-diisocyanate (TDI), isophorone diisocyanate (IPDI), methylene bis(4-cyclohexyl isocyanate) (HMDI), or hexamethylene diisocyanate (HDI) and subsequntly sulfated with a sulfur trioxide reagent. The influence of degree of sulfation was investigated using a polymer. Polyurea glycopolymers with sulfur contents ranging from ∼3% to 15% were prepared from HMDI polymerized with the lactose-containing diamine. The obtained glycopolymers have been characterized using gel permeation chromatography, nuclear magnetic resonance spectroscopy, Fourier transform infrared spectroscopy, and elemental analysis. The blood compatibility of the polymers was determined by measuring the activated partial thromboplastin time, thrombin time, and prothrombin time. Poly(HMDI-sL) exhibited higher prolongation of the activated partial thromboplastin time than other diisocyanate-containing sulfated glycopolymers, and higher degrees of sulfation result in prolonged clotting times. The polymers ability bind thrombin in the presence and absence of antithrombin III was measured using a coloremtric thrombin inhibition assay and the results followed the trend observed in the activated partial thromboplastin time assay.

 Please wait while we load your content...
Please wait while we load your content...