A conformational locking strategy in linked-acceptor type polymers for organic solar cells†
Abstract
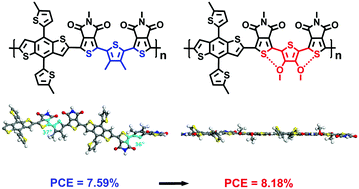
In this paper, two novel linked-acceptor type D–A conjugated polymers POP and POM have been synthesized by Stille polymerization as donor materials for polymer solar cells (PSCs). The concept of introducing intramolecular noncovalent conformational locks into the main chain has been implemented to improve the coplanarity of the linked-acceptor polymers using alkyl substituted thiophenes as π bridges. In this paper, the alkyl side chain substituted in the thiophene bridge has been replaced with an alkoxy chain, in which the oxygen atoms on the side chain and the sulfur atoms on the neighbor thiophene unit could form a coulombic interaction and expand the conjugation degree of the polymers. As a result, the new polymer POP shows better planarity, absorbance ability and processability than that without conformational locks. Although the open-circuit voltage has a small decrease due to the stronger electron-donating nature of the alkoxy group, the fill factor and the current density values have been increased and the resulting best power conversion efficiency has been increased up to 8.18%. This work will become an impactful extending work of linked-acceptor type conjugated polymers and the result suggests that this conformational locking strategy might be a very promising method for the design and construction of novel highly efficient donor materials.

 Please wait while we load your content...
Please wait while we load your content...