Synthesis of highly refractive and highly fluorescent rigid cyanuryl polyimines with polycyclic aromatic hydrocarbon pendants†
Abstract
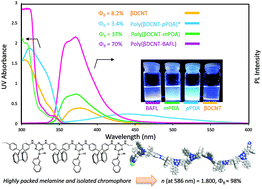
A series of rigid cyanuryl polyimines, polyguanamines (PGs) bearing polycyclic aromatic hydrocarbon pendants were successfully synthesized from 2-substituted 4,6-dichloro-1,3,5-triazine and aromatic diamine monomers used in conventional solution polymerization. In addition, their thermal and optical properties were also investigated. All polymers showed high thermostabilities (Tg ∼ 320 °C, Td5 (N2) ∼ 466 °C, residue at 800 °C under nitrogen ∼69.0%) and adequate solubilities in polar organic solvents. Films prepared by the solvent-cast method showed good transparencies, which mainly depended on the diamine structure as opposed to the dichloride moiety. The refractive indices at the D-line (589 nm) of the PG films were unexpectedly high, between 1.677 and 1.800, compared to those of common organic optical polymeric resins. The incorporated melamine moieties afforded effective dense packing with the polycyclic aromatic hydrocarbon groups filling the free spaces between rigid polymer chains, resulting in unusually high refractive indices. The PG polymer solution in N-methylpyrrolidone showed strong blue fluorescence (371–471 nm) with a quantum yield of up to 98%.

 Please wait while we load your content...
Please wait while we load your content...