One-pot synthesis of poly(vinylidene fluoride) methacrylate macromonomers via thia-Michael addition†
Abstract
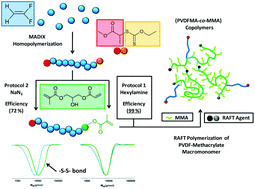
This study presents a new synthetic route to prepare original PVDF macromonomers and PVDF-based architectures. A poly(vinylidene fluoride), PVDF, synthesized using MADIX controlled polymerization in the presence of O-ethyl-S-(1-methoxycarbonyl)ethyldithiocarbonate was chemically modified via two strategies and fully characterized. Using a one-pot procedure, the xanthate end-groups of the PVDF were converted into thiols which were immediately added onto the acrylate moieties of 3-(acryloyloxy)-2-hydroxypropyl methacrylate (AHPMA) via regioselective thia-Michael addition to form new PVDF-MA macromonomers. Two methods of elimination of the thiocarbonylthio group were tested and compared: aminolysis, and elimination using sodium azide. These reactions were thoroughly examined via1H and 19F NMR spectroscopy and SEC-HPLC. The aminolysis procedure was shown to give better coupling efficiency and better-defined macromonomers. The PVDF-MA macromonomers with the highest functionality were further polymerized by RAFT. The RAFT homopolymerization of PVDF-MA revealed that a non-negligible amount of macromonomers did not react. In contrast RAFT copolymerization of PVDF-MA and MMA resulted in the total conversion of the macromonomers and allowed the synthesis of novel methacrylic copolymers and block copolymers.

 Please wait while we load your content...
Please wait while we load your content...