Novel naphthalimide–amine based photoinitiators operating under violet and blue LEDs and usable for various polymerization reactions and synthesis of hydrogels†
Abstract
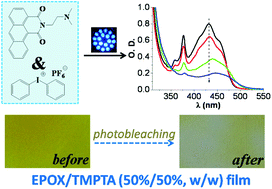
A series of naphthalimide derivatives containing tertiary amine groups (DNNDs) have been prepared. Some of these derivatives (e.g. DNND3, DNND4 and DNND5) exhibit interesting shifted absorption so that they can be utilized as versatile photoinitiators upon exposure to various violet and blue LEDs (385, 405, 455 and 470 nm). They are particularly efficient for the cationic photopolymerization of an epoxide and the free radical photopolymerization of an acrylate. The thiol–ene photopolymerization, as well as the synthesis of interpenetrating polymer networks (of epoxide/acrylate blend) IPNs, is feasible. Remarkably, the production of hydrogels can also be easily achieved using a DNND derivative after inclusion in a cyclodextrin cavity. The photochemical mechanisms have been comprehensively investigated by steady state photolysis, Electron Spin Resonance (ESR), fluorescence, electrochemistry and laser flash photolysis and discussed in detail.

 Please wait while we load your content...
Please wait while we load your content...