Comb-like poly(l-cysteine) derivatives with different side groups: synthesis via photochemistry and click chemistry, multi-responsive nanostructures, triggered drug release and cytotoxicity†
Abstract
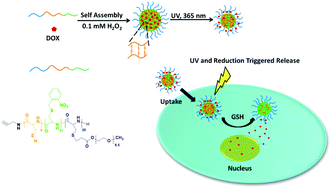
A series of comb-like graft polypeptides having different side groups and tunable grafting ratios were prepared by sequential photocleavage reactions and Michael-type thiol–ene addition, as fully characterized by 1H NMR, gel permeation chromatography, FT-IR, and circular dichroism spectroscopy. The grafting ratio of the resulting polypeptides was close to the photolysis ratio of o-nitrobenzyl derived poly(L-cysteine) precursors. Both electrolytic acrylic acid (AA) and 2-(dimethylamino) ethyl acrylate (DMAEA) produced a larger disturbance on the ordered conformations than neutral poly(ethylene glycol) (PEG) methyl ether acrylate although the molecular weight and size of AA and DMAEA are much smaller than those of PEG. The polypeptide vesicles and micelles with a neutral or electrolytic corona could be facilely fabricated from these side group modified poly(L-cysteine)s, and the polypeptide vesicles exhibited both light- and redox-sensitivity in aqueous solution. As characterized by the MTT assay, flow cytometry, and CLSM, the anticancer drug doxorubicin (DOX) loaded nanoparticles quickly entered into HeLa cells and presented photo- and reduction-triggered cytotoxicity. The half maximal inhibitory concentration of HeLa cells (IC50) for the irradiated nanoparticles dropped to 0.40 μg DOX equiv. per mL compared to 1.27 μg DOX equiv. per mL for the non-irradiated sample; however, the IC50 for the irradiated sample increased about 1.5-fold after the BSO inhibitor treatment. Importantly, this work not only establishes a facile method for the preparation of comb-like graft polypeptides by the combination of photochemistry and thiol–ene click chemistry, but also provides a promising platform for on-demand nanomedicine and cancer therapy.

 Please wait while we load your content...
Please wait while we load your content...