The enhanced performance of fluorinated quinoxaline-containing polymers by replacing carbon with silicon bridging atoms on the dithiophene donor skeleton
Abstract
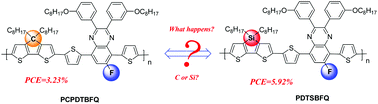
Fluorinated quinoxaline units are attractive acceptor blocks for building low band gap photovoltaic polymers. In this contribution, two novel fluorinated quinoxaline-based copolymers, PCPDTBFQ and PDTSBFQ, have been successfully synthesized by combination of cyclopentadithiophene (CPDT) or dithienosilole (DTS) donor blocks in the polymeric backbones. The bridging atom effect shows a great influence on the absorption properties, energy level, carrier mobility as well as photovoltaic performance. PDTSBFQ with Si bridging atoms shows a little blue-shift in the UV-vis absorption, and a little larger band gap than PCPDTBFQ. However, PDTSBFQ shows a stronger aggregation even in the solution state. Compared with carbon, silica atoms afforded PDTSBFQ with a lower-lying HOMO level, suggesting a high Voc for polymer solar cells (PSCs). PDTSBFQ also exhibits better crystallinity and molecular ordering properties than PCPDTBFQ from the XRD study. The hole-only device based on PDTSBFQ exhibited a higher hole mobility of 1.5 × 10−5 cm2 V−1 s−1 than that of 4.1 × 10−6 cm2 V−1 s−1 for PCPDTBFQ. Conventional PSCs were fabricated to investigate the bridging atom effect on the photovoltaic properties of these two copolymers. Different interfacial layers (IFLs) of Ca and PFN had been tried to optimize the performance of the fabricated PSCs. When using a Ca/Al top electrode, PCPDTBFQ and PDTSBFQ devices showed PCEs of 2.16% and 4.43%, respectively. The better performance of the PDTSBFQ device can be attributed to the improvements in its Voc, Jsc and FF values just by choosing silica bridging atoms. After replacing the Ca/Al with a PFN/Al cathode, the performance of both PCPDTBFQ and PDTSBFQ devices was enhanced largely. The PDTSBFQ device achieved the highest PCE of 5.92% with a Voc of 0.77 V, a Jsc of 12.25 mA cm−2 and an FF of 0.63. The primary results gave a simple but efficient strategy to design high performance dithiophene-quinoxaline polymer donors for organic solar cell applications.

 Please wait while we load your content...
Please wait while we load your content...