Poly(alkylene itaconate)s – an interesting class of polyesters with periodically located exo-chain double bonds susceptible to Michael addition†
Abstract
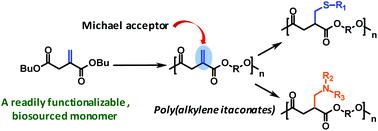
Itaconic acid is a bio-sourced dicarboxylic acid that carries a double bond; although several reports have dealt with the radical-initiated chain polymerization of dialkyl itaconates, only a few studies have utilized it as a di-acid monomer to prepare polyesters. In this study, we demonstrate that dibutyl itaconate can be melt-condensed with aliphatic diols to generate unsaturated polyesters; importantly, we show that the double bonds remain unaffected during the melt polymerization. A particularly useful attribute of these polyesters is that the exo-chain double bonds are conjugated to the ester carbonyl and, therefore, can serve as excellent Michael acceptors. A variety of organic thiols, such as alkane thiols, MPEG thiol, thio-glycerol, derivatized cysteine etc., were shown to quantitatively Michael-add to the exo-chain double bonds and generate interesting functionalized polyesters. Similarly, organic amines, such as N-methylbenzylamine, diallyl amine and proline, also add across the double bond; thus, these poly(alkylene itaconate)s could serve as potentially bio-benign polyesters that could be quantitatively transformed into a variety of interesting and potentially useful functionalized polymers.

 Please wait while we load your content...
Please wait while we load your content...