Synthesis and migration insertion polymerization (MIP) of CpFe(CO)2(CH2)6PPh2 (FpC6P) for PFpC6P: macromolecule stability, degradability and redox activity†
Abstract
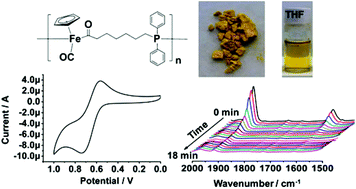
CpFe(CO)2(CH2)6PPh2 (FpC6P) is synthesized as a monomer for bulk migration insertion polymerization (MIP). Due to the presence of a longer alkyl spacer in FpC6P, intramolecular cyclization reactions, which are observed during the MIP of CpFe(CO)2(CH2)3PPh2 (FpC3P), were completely suppressed under the conditions of the polymerization. The resultant PFpC6P is soluble in common organic solvents and stable enough for GPC analysis. However, the polymer degrades in solution over a few days because the PFpC6P chain is constructed from relatively weak metal coordination bonds. In the presence of an oxidant, e.g. H2O2, degradation of the polymers was accelerated. When the solution was exposed to LED light (wavelength: 400–410 nm), degradation was completed in 20 minutes with CO release first followed by the dissociation of phosphine. DSC analysis indicates that PFpC6P has a Tg of ca. 68 °C, which is lower than that of PFpC3P (ca. 99 °C). TGA analysis reveals that PFpC6P is thermally stable up to 170 °C and leaves a ca. 35.2% char yield at 676 °C after three stages of weight loss. CV data shows that the polymer in DMF has reversible redox activity, with an oxidation peak at 0.74 V and a reduction peak at 0.56 V (relative to a Ag electrode).

 Please wait while we load your content...
Please wait while we load your content...