Side chain thiol-functionalized poly(ethylene glycol) by post-polymerization modification of hydroxyl groups: synthesis, crosslinking and inkjet printing†
Abstract
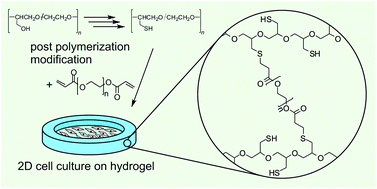
Polymers with a poly(ethylene glycol) backbone and mercaptomethyl side chains were synthesized by post-polymerization modification of hydroxymethyl side chains in three steps. As the starting point of the synthetic route, linear copolymers of ethylene oxide and glycidol with molar contents of glycidol repeating units of approximately 20, 40, 60, 80 and 100% were used. The polymer-bound hydroxyl groups were converted to thiol groups in three steps, comprising tosylation, introduction of a triphenylmethyl protected thiol and thiol deprotection by acid treatment. The degree of thiol-functionalization was controlled by the degree of functionalization of the starting material. The degree of conversion of hydroxyl groups to thiol groups determined by 1H NMR spectroscopy was quantitative for copolymers with approximately 20 and 40% glycidol repeating units and 92, 81 and 87% for copolymers with approximately 60, 80 and 100% glycidol repeating units, respectively. Exemplarily, poly(glycidylthiol) obtained by conversion of poly(glycidol) was crosslinked with poly(ethylene glycol) diacrylate (PEG-DA) to yield hydrogels which supported adhesion and proliferation of human fibroblasts 48 h after cell seeding. Spatially defined and surface attached gel structures were fabricated by subsequent inkjet printing of poly(glycidylthiol) and PEG-DA solutions onto acrylated glass slides.

 Please wait while we load your content...
Please wait while we load your content...