Novel blue-emitting carboxyl-functionalized poly(arylene ether nitrile)s with excellent thermal and mechanical properties†
Abstract
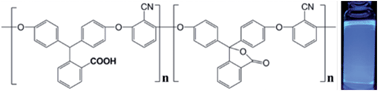
A series of novel carboxyl-functionalized poly(arylene ether nitrile)s (CPAENs) were synthesized via nucleophilic substitution polycondensation reactions of 2,6-dichlorobenzonitrile with carboxyl-functionalized phenolphthalin and diphenol compounds, using N-methyl-2-pyrrolidone (NMP) as solvent in the presence of anhydrous potassium carbonate. The resulting CPAENs exhibited high glass transition temperatures ranging from 181 °C to 251 °C, and were thermally stable up to 400 °C under either nitrogen or air atmospheres. The incorporation of phenolphthalin-units into the polymer chain imparted an improved solubility of CPAENs in organic solvents, such as NMP, N,N-dimethylformamide, and tetrahydrofuran. The CPAENs were amorphous and can be readily cast into transparent films with a tensile strength of 75.1–104.7 MPa and a tensile modulus of 2.6–3.2 GPa. All CPAENs displayed a highly intense UV absorption in the wavelength range of 280–330 nm and a characteristic blue-emitting fluorescence under the UV irradiation.

 Please wait while we load your content...
Please wait while we load your content...