Synthesis of a poly(N-isopropylacrylamide) charm bracelet decorated with a photomobile α-cyclodextrin charm†
Abstract
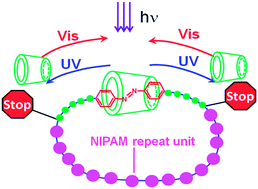
Cyclic poly(N-isopropylacrylamide) with azobenzene inserted in the main chain (azo-c-PNIPAM, Mn 8800 g mol−1) was synthesized by the ‘click’ ring closure of α-azobenzene ω-azido poly(N-isopropylacrylamide) obtained by reverse addition–fragmentation transfer (RAFT) polymerization of NIPAM with an azobenzenyl-substituted chain transfer agent. Taking advantage of the azobenzene–α-cyclodextrin inclusion complex and conducting the ring closure in the presence of excess α-cyclodextrin (α-CD), we prepared azo-c-PNIPAM with an interlocked α-CD of topology reminiscent of a single-charm bracelet. The polymers were characterized by gel permeation chromatography, 1H NMR spectroscopy, UV-visible absorption spectroscopy, and FTIR spectroscopy. 2D NOESY spectroscopy confirmed that the azobenzene group of the ring was inserted into the α-CD cavity. Trans to cis isomerization of the azobenzene group was induced by irradiation at 365 nm. The cis-azobenzene moiety was expelled from the α-CD cavity, as indicated by a hypsochromic shift of the π–π* electronic transition, corroborated by circular induced dichroism (CD) spectroscopy, 1H NMR and 2D NOESY spectroscopy. The reverse cis to trans isomerization induced by irradiation at 440 nm triggered the re-insertion of the azobenzene group into the α-CD cavity.

 Please wait while we load your content...
Please wait while we load your content...