A polymeric chain extension driven by HSCT interaction†
Abstract
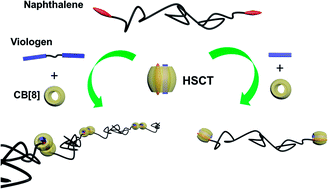
In this paper, targeting a high-molecular-weight supramolecular polymer, the chain extension of low-molecular-weight polymers (LMWPs) is achieved via the HSCT (Host-Stabilized Charge Transfer) of CB[8] (cucurbit[8]uril). Here, a combination of ditopic viologen and CB[8] serve as a “supramolecular chain extender” for the first time, to connect the naphthalene (Np)-ended LMWP PDMA or PNIPAm. UV-vis spectra, ITC and NMR prove that the guest Np connected to the LMWP does not affect its complexation with guest viologen and CB[8]. The viscosity measurements clearly demonstrate the formation of a supramolecular polymer, as the viscosity of the LMWP PDMA (DP 52) after complexation exceeds that of PDMA (DP 300). Np-ended LMWP PNIPAm shows a similar chain extension. The resultant PNIPAm supramolecular polymer shows peculiar LCST behavior with a remarkably different variation in concentration from its polymeric precursor.

 Please wait while we load your content...
Please wait while we load your content...