A facile transetherification route to polysulfone-poly(ethylene glycol) amphiphilic block copolymers with improved protein resistance†
Abstract
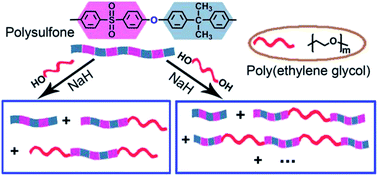
A transetherification reaction between polysulfone (PSf) and poly(ethylene glycol) (PEG) alkoxide was proposed and explored for the preparation of polysulfone-poly(ethylene glycol) (PSf–PEG) amphiphilic block copolymers. The reaction efficiency and product structure were investigated by GPC, FTIR and 1H NMR. A bimolecular nucleophilic substitution mechanism (SN2) was proposed and verified by analyzing the linkage and terminal structures of the products. The molecular weight of copolymers determined by GPC was coincident with theoretical value. The mechanical properties of PSf–PEG were preserved by using HO–PEG–OH of high molecular weight. TGA and DSC were adopted to characterize the thermal properties of prepared copolymers. The good non-specific protein absorption resistance of PSf–PEG materials renders them desirable candidates for biomedical applications.

 Please wait while we load your content...
Please wait while we load your content...