Fluorene-functionalized aliphatic polycarbonates: design, synthesis and aqueous self-assembly of amphiphilic block copolymers†
Abstract
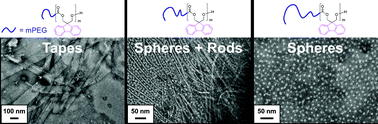
In this study, a fluorene-functionalized aliphatic cyclic carbonate monomer, spiro[fluorene-9,5′-[1,3]-dioxan]-2′-one (F-TMC) was synthesized, and organo-catalytic ring opening polymerization of F-TMC was explored with 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU). By using mPEG-OH with a variety of different molecular weights (1.6–10.0 kDa) as macro-initiators, well-defined amphiphilic diblock copolymers mPEGn-b-P(F-TMC)m, were readily synthesized with relatively low polydispersity indices, encompassing hydrophilic PEG weight fractions (f) from 0.24 to 0.67. Depending upon the specific amphiphilic balance, under aqueous conditions, these block copolymers self-assembled to form unique nanostructures such as tapes, elongated micelles and spherical micelles. The electro-active nature of the fluorene moieties combined with the biodegradable polycarbonate platform could render these nanostructures useful as novel materials for the encapsulation and release of active ingredients through π–π and hydrophobic interactions.

 Please wait while we load your content...
Please wait while we load your content...