A new strategy of post-polymerization modification to prepare functionalized poly(disubstituted acetylenes)†
Abstract
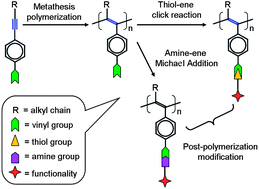
Two disubstituted acetylenes bearing a vinyl group at one end (M1 and M2) were synthesized and polymerized by WCl6–Ph4Sn catalyst. The expected poly(disubstituted acetylenes) PDSAs (P1 and P2) were obtained in high yields. Both P1 and P2 have reactive vinyl groups on their side chains, thus they were used as precursors to be subsequently modified with a mercapto compound through the thiol–ene click reaction to produce the novel PDSAs (P1S and P2S) in good yield. The chemical structures of the polymers were carefully characterized by standard spectroscopic methods such as gel permeation chromatography (GPC), NMR, NMR and FTIR techniques, and satisfactory data were collected. The post-polymerization modification of P1 took a long reaction time (3 days) to convert P1 to P1S because the ene-functionality at the end of side chain of P1 links to a saturated alkyl segment. By using an activated ene-functionality (a,ß-unsaturated vinyl), the modification of P2 to P2S took only 1 day under mild conditions. Moreover, the activated end-ene group allowed the post-polymerization modification of P2 by Michael addition, which was confirmed using butylamine as the representative amine compound and the characterization data indicated the validity of the expected P2N. The thermal analysis results indicated that the modified polymers were highly stable thermally with a decomposition temperature over at least 240 °C, except for P2N, which showed lower stability due to unstable imine groups. Meanwhile, it was found that the modified polymers P1S and P2S were fluorescent and showed similar emission efficiency to their precursors P1 and P2. These results indicated that the thiol–ene click reaction and Michael addition reaction are accessible routes for post-polymerization modification to generate novel functional PDSAs.

 Please wait while we load your content...
Please wait while we load your content...