Micellar-cluster association of ureidopyrimidone functionalized monochelic polybutadiene†
Abstract
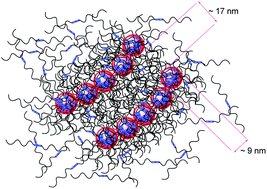
A series of monochelic polybutadienes with a ureidopyrimidone terminal group (MPBd-UPy) has been synthesized using anionic polymerization and chain-end transformation. Monochelic polybutadiene with two UPy terminal groups, MPBd-(UPy)2, has also been synthesized to study the effect of chain-end concentration on hydrogen bonding association. The transformation of the precursor ω-hydroxyl terminated polybutadiene into the ureidopyrimidone functionality via the reaction with 1-(6-isocyanatohexyl)-3-(6-methyl-4-oxo-1,4-dihydropyrimidin-2-yl) urea (UPy-NCO) turns free-flowing liquid samples into a semi-solid, viscous material, due to the well-known quadruple hydrogen bonding ability of UPy. The dimerization association of MPBd-UPy has been studied thoroughly using size exclusion chromatography, intrinsic viscosity and dynamic light scattering. Solutions of MPBd-UPy exhibit a high Huggins parameter, 0.40 < KH > 0.63, depending on the molecular weight and the number of UPy groups at the chain-end. Studies reveal that along with hydrogen bonded dimers, (MPBd-UPy)2, they also aggregate intermolecularly to form star-like micelles that are in equilibrium with the dimers. The presence of multiple populations with Rh as high as 700 nm, which decreases with increasing temperature in toluene has been identified. The UPy aggregated micellar clusters have distinct endothermic transitions in the solid-state and atomic force microscopy studies show the presence of micellar-clusters forming a well-defined assembly of fibrous like parallel lines on a mica surface.

 Please wait while we load your content...
Please wait while we load your content...