Synthesis and solid state helix to helix rearrangement of poly(phenylacetylene) bearing n-octyl alkyl side chains†
Abstract
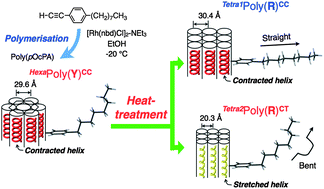
Highly stereoregular polymerisations of p-n-octyphenylacetylene (pOcPA) were performed using a [Rh(norbornadiene)Cl]2-triethylamine catalyst in ethanol at −20 and 25 °C to afford yellow and orange polymers, Poly(Y) and Poly(O), in yields of 64 and 99%, respectively. The XRD patterns of Poly(Y) showed a hexagonal columnar crystal with a contracted cis–cisoid helix, HexaPoly(Y)CC. The XRD pattern of Poly(O) matched that of Poly(Y) when heated to 80 °C. The heat treatment of HexaPoly(Y)CC at 100 °C generated two tetragonal crystals: Tetra1Poly(R)CC, containing contracted cis–cisoid helices, and Tetra2Poly(R)CT, containing stretched cis–transoid helices. The helical diameters of HexaPoly(Y)CC before and after heat treatment were estimated using XRD and were consistent with the results of MMFF 94 calculations, although the n-octyl alkyl chains of HexaPoly(Y)CC and Tetra2Poly(R)CT did not have linear alkyl chains; a bend in the chains was confirmed by 13C CP-MAS NMR. When HexaPoly(Y)CC was heated to 100 °C in the solid phase, the λmax in the diffuse reflective UV-vis spectra shifted from 448 nm to 565 nm. Furthermore, the endothermic transition for HexaPoly(Y)CC occurred at 100 °C in the DSC trace. Therefore, these data corroborated the assertion that HexaPoly(Y)CC thermally converted into Tetra1Poly(R)CC and Tetra2Poly(R)CT.

 Please wait while we load your content...
Please wait while we load your content...