RAFT copolymerization of acid chloride-containing monomers†
Abstract
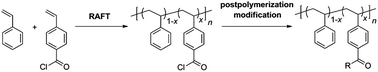
Controlled copolymerization of acryloyl chloride (AC), methacryloyl chloride (MAC), and vinylbenzoyl chloride (VBC) with styrene via the reversible addition–fragmentation chain transfer (RAFT) process was investigated. Copolymerization was conducted in 1,4-dioxane at 60 °C using azobisisobutyronitrile as an initiator and S-1-dodecyl-S′-(R,R′-dimethyl-R′′-acetic acid) trithiocarbonate as a chain transfer agent (CTA). The reactive copolymer was obtained by precipitating in hexanes. Methyl ester analogues of the reactive polymers were obtained by precipitating in methanol for analytical purposes and their 1H nuclear magnetic resonance spectroscopy and size exclusion chromatography analyses indicated that the best control was achieved for P(S-co-VBC) produced by copolymerization of styrene and VBC. Kinetics of the copolymerization of styrene and VBC was consistent with the RAFT mechanism. Reactive block polymers consisting of the P(S-co-VBC) block were also readily prepared using a macromolecular chain transfer agent. P(S-co-VBC) was successfully functionalized by reaction with alcohols or amines to form ester or amide linkages demonstrating its utility for the postpolymerization modification approach.

 Please wait while we load your content...
Please wait while we load your content...