Synthesis and characterization of fluorinated polyionomers. Part I: polyperfluoro-sulfonylethoxy propylene vinyl ether sulfonimides containing aryl sulfonic acids†
Abstract
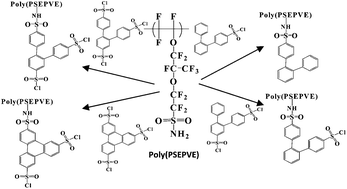
The synthesis and chemistry associated with the preparation of polysulfonimide polyionomers containing aryl sulfonic acids with high charge densities and various solubilities is reported.

 Please wait while we load your content...
Please wait while we load your content...