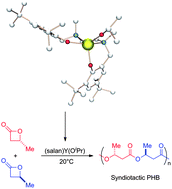
Synthesis of aliphatic polyesters has been studied intensively due to their biocompatible and biodegradable properties and their applications in medical and agricultural fields. Among them, polyhydroxybutyrate (PHB), which is a naturally occurring polymer, is synthesized by a variety of bacteria and algae. However, this polymer has poor mechanical properties due to its brittleness. Herein is presented a practical route to a highly syndiotactic PHB by way of a one-pot reaction. We report the results of a comprehensive investigation of the newly discovered stereoselective and controlled polymerization of racemic β-butyrolactone (rac-BBL) using an initiator prepared in situ from yttrium(III) isopropoxide Y(OiPr)3, and a bisphenoxide ligand. DFT studies on the alkoxide catalyst reveal that the R and S monomers are almost equivalent for the first ring-opening reaction. The selectivity of the next insertion was scrutinized, demonstrating that the polymerization process is predicted to be stationary (back-side insertion).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...