Preparation and properties of novel oligo(phenylene oxide)-branched cyclophosphazenes
Abstract
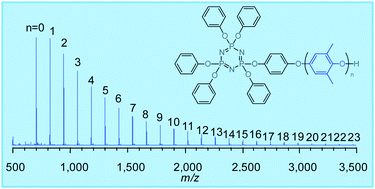
Novel oligo(phenylene oxide)-branched cyclophosphazenes (POPZs) were synthesised by a redistribution reaction of poly(2,6-dimethyl-1,4-phenylene oxide) (PPO) with 4-hydroxyphenoxycyclotriphosphazenes in the presence of benzoyl peroxide (BPO) as a free-radical initiator. The redistribution yielded benzoyl moiety-substituted cyclotriphosphazenes in addition to cyclotriphosphazene-branched oxy-2,6-dimethyl-1,4-phenylene (ODMP) repeating units in the hydroxyphenoxy group. The benzoyl moieties originated from the BPO and attached to the methyl side group and the terminal hydroxy group of a constitutional ODMP unit. The benzoyl moiety in the cyclophosphazenes was eliminated completely via hydrolysis with NaOMe, followed by chlorination with SOCl2 and reduction with LiAlH4. Both the redistribution and elimination reactions were observed using FT-IR, LC-MS, 1H NMR, 13C NMR, 31P NMR, MALDI-TOF mass spectroscopy, and size exclusion chromatography (SEC). The resulting POPZs were evaluated for their thermal and electrical properties and their flame retardancy. The benzoyl moiety-free POPZs exhibited high thermal stability up to 300 °C and electrical properties such as dielectric constants of 2.56–2.67 and dissipation factors of 0.0044–0.0049.

 Please wait while we load your content...
Please wait while we load your content...