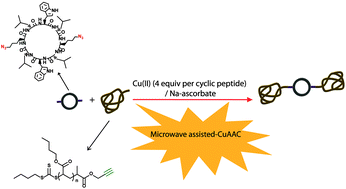
We have investigated the copper mediated azide–alkyne cycloaddition (CuAAC) of chain-end alkyne-functionalised polymeric chains onto azide-functionalised cyclic peptides. We observe that steric effects limit the efficiency of the conjugation reactions when attempting to ligate four polymeric chains to one cyclic peptide. A maximum of ca. 50% efficiency is obtained, leading to a product containing a mixture of two, three and four-arm conjugates. On the other hand, conjugation of chain-end alkyne-functionalised polymers to a two-arm azido cyclic peptide occurred with 100% efficiency, independently of the polymeric chain length (degree of polymerisation varying from 16 to 108). In this case, optimal conjugation conditions required 1.025 equivalents of polymer, to account for the polymer molecular weight distribution, and two equivalents of CuSO4 relative to azide, in DMF/TFE (1 : 1 v/v) for 15 minutes under microwave irradiation. Independent of the efficiency of the conjugation reaction, all the conjugates were observed to form nanotubes in solution.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...