Synthesis of DNA intercalator–immobilized cyclodextrin and interaction with double-stranded DNA: Utilization of DNA–cyclodextrin conjugated material as an environmental remediation material
Abstract
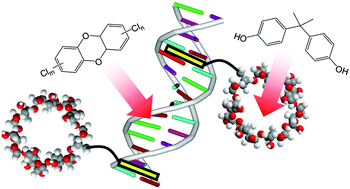
Intercalator–immobilized cyclodextrin (PβCD) was synthesized by the linking of various methylene chains between psoralen, one of the DNA intercalators found in nature, and β-cyclodextrin, a cyclic oligosaccharide. The psoralen part of the PβCD was intercalated into the double-stranded DNA and formed the stable DNA–PβCD conjugated material with UV irradiation at 365 nm. The DNA–PβCD conjugated material possessed both the functions of double-stranded DNA, such as intercalation, and cyclodextrin, such as encapsulation into the intramolecular cavity. As a result, planar structure-containing harmful compounds, such as dioxin and polychlorobiphenyl (PCB) derivatives, were accumulated by the intercalation of double-stranded DNA. Non-planar structure-containing compounds, such as bisphenol A and diethylstilbestrol, were accumulated by the encapsulation of cyclodextrin. Additionally, DNA–PβCD conjugated material can accumulate various harmful compounds from an aqueous multi-component solution, which contains the model endocrine disruptors. Furthermore, the accumulative amount of harmful compounds increased with the length of the methylene linker. Therefore, the DNA–PβCD conjugated material may have the potential for use in environmental remediation, such as the accumulation of harmful compounds from factory effluent.

 Please wait while we load your content...
Please wait while we load your content...