Preparation of hydrophobic helical poly(N-propargylamide)s in aqueous medium via a monomer/cyclodextrin inclusion complex
Abstract
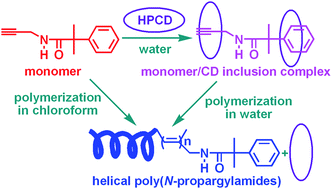
We present a facile approach for the preparation of hydrophobic helical poly(N-propargylamide)s in aqueous medium instead of toxic and volatile organic

 Please wait while we load your content...
Please wait while we load your content...